Hydrophobins nanovehicles systems for the protected delivery of sensitive hydrophobic nutraceuticals
Hydrophobins are small cysteine-rich, amphipathic proteins, present in large amounts in fungal cell walls. Due to their amphiphilic nature and self-assembly properties, hydrophobins show promise as highly effective surfactants, emulsifiers and nanoencapsulating agents in food systems. We examined the possibility to enrich clear beverages, such as mineral water with hydrophobic nutraceutical compound by using the new delivery system of hydrophobin.
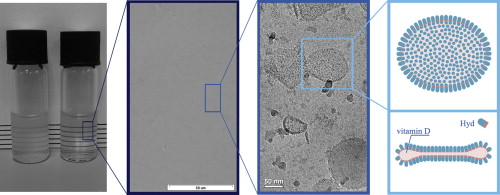
Hyd tend to self- assemble in aqueous solution thus bind the hydrophobic substance VD3. VD3-Hyd complexes are nanoscale, and their aqueous solution is transparent, thus they may be suitable for enrichment of clear beverages. Moreover, Hyd provide excellent protection to VD3 against degradation. (Israeli-Lev et al (2014))
Controlling hydrophobic bioactive nanocrystal size, morphology and bioavailability by harnessing proteins.
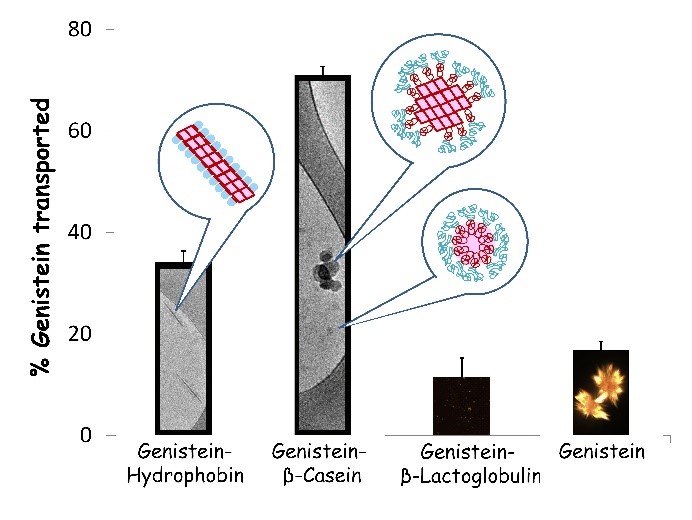
Protein-surface and protein-crystal interactions are important in many areas of technology including drug and nutraceutical delivery, as many bioactives are highly hydrophobic and tend to crystallize, resulting in poor bioavailability. The improved ability to control lipophilic bioactive nanocrystal formation and dispersibility can increase colloidal stability, and open new ways to control the release of incorporated bioactives and their bioavailability. We compared three model proteins: b-casein, hydrophobin, and b-lactoglobulin, representing different structural groups of proteins, and assessed their functionality in preventing crystal growth, using genistein as a model hydrophobic crystallizing bioactive. Dynamic light scattering, polarized light microscopy and cryo-TEM showed that b-lactoglobulin, hydrophobin and b-casein, respectively inhibit genistein crystal growth in aqueous solution in increasing order of efficacy. Protein structure determines the mechanism and the efficacy by which it affects crystal growth and morphology: b-lactoglobulin, a rigid globular protein with an inward facing hydrophobic domain, indirectly suppresses crystallization by binding and reducing concentration of free hydrophobic compound molecules. Hydrophobin, a rigid globular protein with a flat external hydrophobic domain, adheres to the surface of certain crystal faces, limiting growth in the perpendicular directions. b-casein, a rheomorphic protein with an external hydrophobic domain, adheres to different crystal faces nonspecifically, thereby blocking growth in all directions. Consequently, an inverse correlation was observed between nanocrystal size and in vitro bioavailability. Based on this study, amphiphilic proteins can be more effectively selected and applied to control crystal growth and morphology of hydrophobic bioactives to improve their delivery and bioavailability in food and drug systems. (Israeli-Lev et al (2017)).